To Inspire Each Other And Create Effective Medicines For Humankind
搜索


NEWS

2024-03-15
Biotheus Enters Into Strategic Partnership with BioNTech to Develop and Commercialize Bispecific Antibody Candidate Targeting PD-L1 and VEGF in Multiple Solid Tumor Indications
2023-11-06
Biotheus Announces Strategic Research Collaboration and Worldwide License Agreement with BioNTech
2023-07-20
Biotheus has entered into a license and collaboration agreement with Hansoh Pharma for its EGFR/MET bispecific antibody in the Greater China territory
2022-11-15
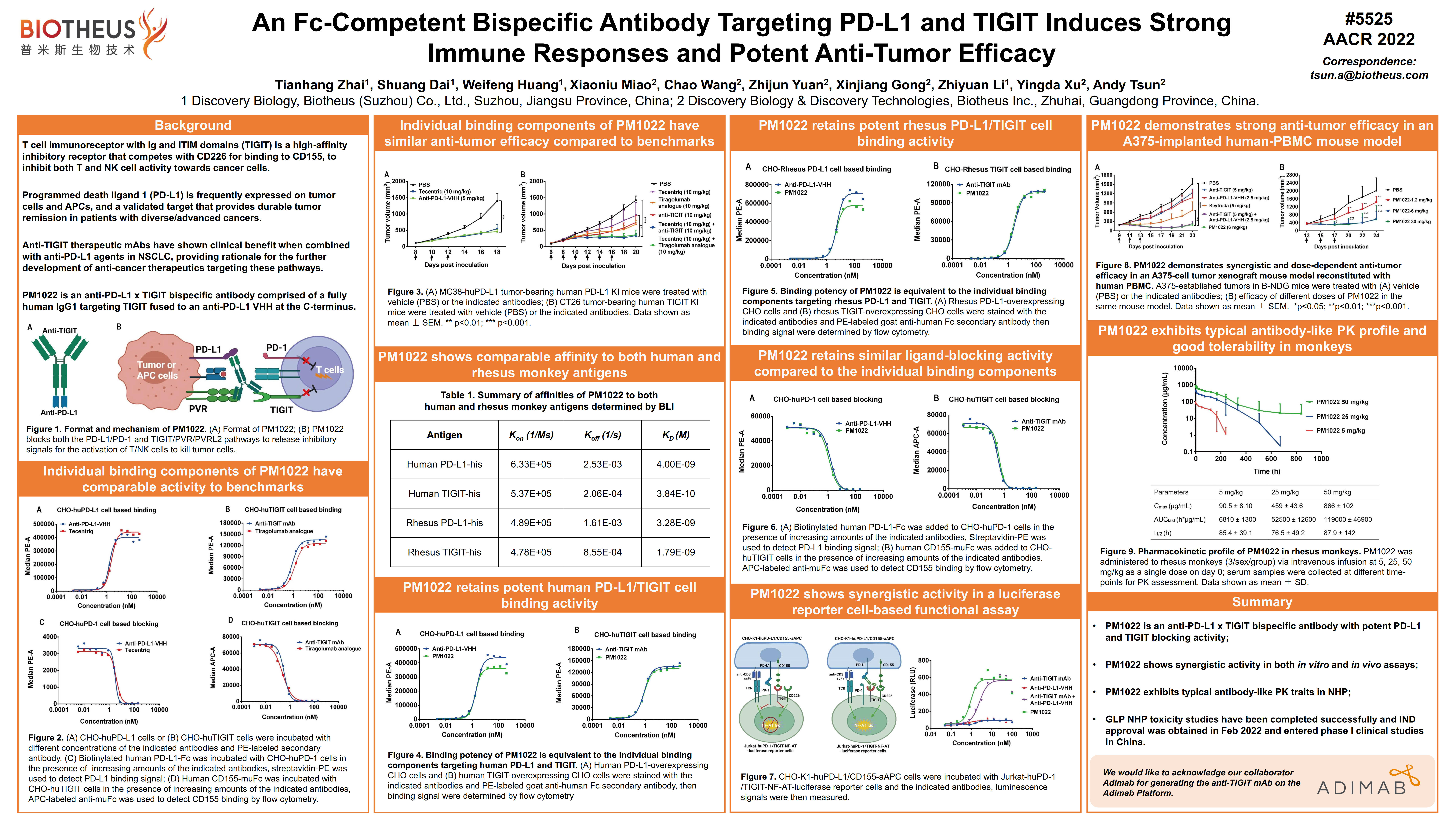
Biotheus Shares Latest Research & Development Updates on Multiple Programs During AACR, 2022 and SITC, 2021
2022-04-15
Biotheus' Bifunctional Therapeutic PM8001 has been Approved by the USFDA to Enter Phase II Studies
2021-12-03

Xiaoniu Miao, Director Antibody R&D of Biotheus:R&D of Antineoplastic New Drugs Accelerated by over USD100 million Financing
2021-05-19

Encouraging the Power of Innovation|The Fifth Medical and Health Investment Bonjour List 2021 Announced
2021-03-26

Biotheus Announces the Closing of a New Round of Financing co-led by General Atlantic and IDG Capital
2021-03-03

Biotheus and Kintor Reached a Strategic Cooperation Agreement on the Development of Biological Drugs
2020-10-20
ABOUT BIOTHEUS

Our mission at Biotheus is "to inspire each other and create effective drugs for humankind", focusing on the discovery and development of novel drugs to treat cancer and inflammatory diseases. We have a rich pipeline of over 20 products, with several products that are currently undergoing clinical trials in China. Our current aim is to expand our clinical research activities worldwide.