

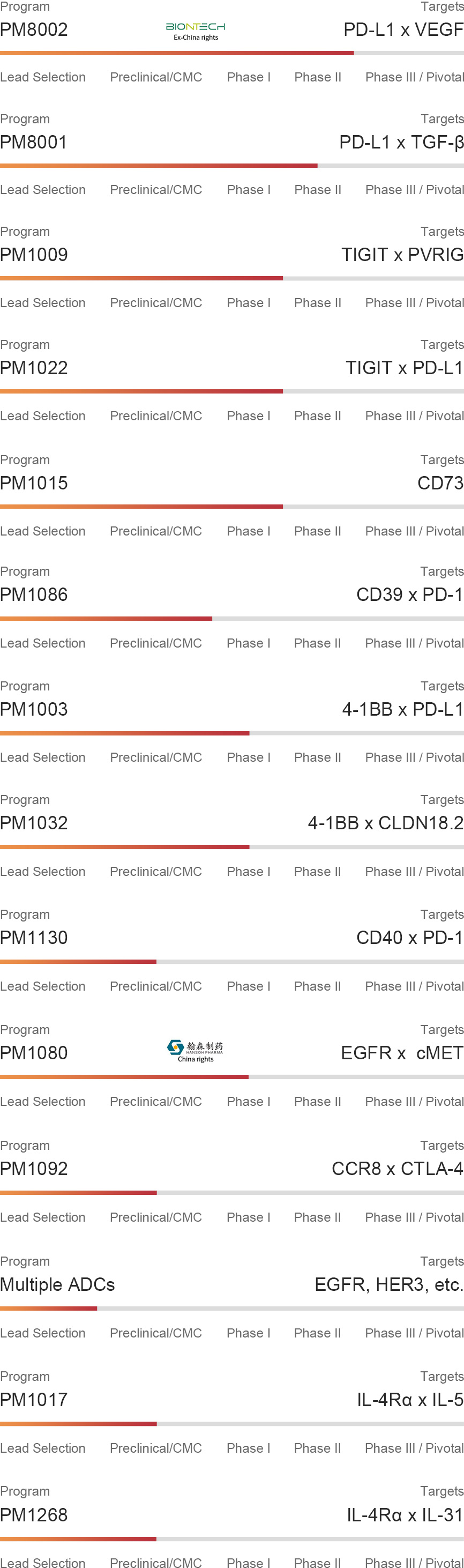
Antibody Pipeline

-
PM8002 is a next generation PD-(L)1-based bispecific that blocks the PD-L1 and VEGF pathways. By adopting PD-L1-based enrichment of the PD-L1-targeting arm, VEGF neutralization is focussed to the PD-L1+ TME to increase local efficacy and reduce off-tumour/on-target toxicity related to VEGF neutralization. By targeting these two synergistic clinically-validated targets, PM8002 can serve as a critical backbone for future combination therapies.
-
Transforming frowth factor-β is upregulated in tumors and changes from tumor-suppressive to tumor-promoting activity. PM8001 is a PD-L1 inhibitor that neutralizes the activity of TGFβ simultaneously. By enriching TGFβ neutralization into the tumor microenvironment, potential off-target effects of TGFβ neutralization may be limited whilst providing synergistic blockade.
-
PM1009 is a next generation checkpoint inhibitor to the PVR-TIGIT axis by inhibiting two non-redundant checkpoints TIGIT and PVRIG. In preclinical studies, improved tumour inhibition was found in combination with PD-(L)1 inhibitors compared to individual agents that target TIGIT or PVRIG alone. Through Fc-characterization in preclinical tumor models, Fc function has been retained for PM1009 on an IgG1 backbone.
-
PM1022 is a bispecific that targets PD-L1 and TIGIT simultaneously. Preclinical studies have shown that Fc function potentiates the therapeutic activity of anitbodies that target TIGIT and PD-L1. PM1022 not only blocks PD-L1 and TIGIT binding to their interaction partners, but effector Fc function allows for killing towards PD-L1-positive tumor cells and TIGIT-postive Tregs.
-
CD73 is highly expressed in tumors and is an ectonucleotidase that drives the conversion of AMP to immunosuppressive adenosine. PM1015 is a potentially best-in-class antagonist of CD73 that differentiates from other competitor agents by blocking membranous and soluble CD73 to maximal levels without hook effects. The reduction of adenosine levels by inhibiting CD73 removes a major pro-tumor pathway for improving treatment of cancer patients.
-
CD39 is highly expressed in the TME and is an ectonucleotidase that converts ATP to AMP. This reduces local ATP levels and promotes the degradation pathway towards adenosine. As ATP stimulates immune reactions and adenosine is an immunosuppressive metabolite, the inhibition of CD39 can improve anti-tumor immune activity. PD-1 and CD39 are both expressed on effector T cells, and blockade of both these pathways have been found to synergize in preclinical tumor models. PM1086 is a highly potent inhibitor to PD-1 and CD39 and has improved avidity to T cells that express these markers simultaneously to reinvigorate tumor-specific T cells in the TME.
-
Biotheus' 4-1BB agonist is a humanized VHH that targets a unique membrane-proximal epitope on 4-1BB situated on the CRD4 domain to maintain agonist activity but in turn reduce toxicity risk. Activation of 4-1BB can improve immune effector function and memory. By pairing with an anti-PD-L1 binder, PM1003 can both inhibit PD-L1 function and activate 4-1BB in PD-L1-enriched tumours. Fc function has been depleted to reduce effector function towards PD-L1+ and 4-1BB+ cells and minimize FcR-mediated crosslinking. In preclinical studies, single-agent activity has been observed, and PM1003 may be used for treating multiple solid tumour types.
-
Biotheus' 4-1BB agonist is a humanized VHH that targets a unique membrane-proximal epitope on 4-1BB situated on the CRD4 domain to maintain agonist activity but in turn reduce toxicity risk. Activation of 4-1BB can improve immune effector function and memory. By pairing with an anti-CLDN18.2 binder, PM1032 activates 4-1BB in CLDN18.2-enriched tumours. Fc function has been depleted to minimize FcR-mediated crosslinking. In preclinical studies, single-agent activity has been observed, and PM1032 may be used to treat CLDN18.2+ solid tumours such as gastric and pancreatic cancer.
-
Biotheus' CD40 agonist is a ligand non-blocker and engineered so that they do not rely on Fc-mediated crosslinking for activation of CD40-expressing myeloid cells. This bispecific has been designed to activate CD40 on APCs via PD-1-mediated cross-bridging and cross-linking via antigen-specific T cells that closely interact with APCs. By testing CD40 bispecifics by paring with various T cell markers, PD-1 has been found to have most synergy as a binding pair. Thus, both PD-1 inhibition and CD40 agonism is contained within one molecule in PM1130. PM1130 may be used to treat multiple solid tumor types that could benefit from upregulating antigen presentation.
-
PM1080 is an asymmetric 1+1 IgG-like bispecific antibody targeting EGFR x c-MET. After the fine tuning of the EGFR and c-MET binding domains, PM1080 preferentially interacts with EGFR/c-MET double positive cancers over EGFR single positive cells. This key feature allows PM1080 to potently suppress EGFR/c-MET expressing tumor progression either as a single agent or in combination with EGFR TKIs. Additionally, it significantly reduced conventional anti-EGFR-related toxicity resulting in a higher therapeutical window.
-
Our next-generation Treg depletion assets focus on targeting CCR8, which is highly upregulated on tumour-infiltrating Tregs. To improve specificity in depleting Tregs in the TME, a second arm has been targeted (CTLA-4) to minimize the depletion of CCR8- or CTLA-4-expressing effector cells such as cytotoxic CD4+ and CD8+ T cells. PM1092 has a 1+1 heterodimeric structure, where the affinities of both the CCR8- and CTLA-4-targeting arms have been optimized for binding and activity towards CCR8/CTLA-4 double-positive cells. In preclinical studies, the CCR8 x CTLA-4 bispecific has better safety than other benchmark anti-CTLA-4 mAbs, and performs better than mAbs targeting CCR8 or CTLA-4 alone for tumor inhibition.
-
-
Dupilumab (anti-IL-4Rα) shows overall better efficacy than other biologics in treating severe eosinophilic asthma. However, hyper eosinophilia has been observed in some patients with some experiencing severe side effects, including those resistant to anti-IL-5/IL-5Rα agents. Mepolizumab (anti-IL-5), which blocks the IL-5/IL-5Rα pathway could inhibit IL-5-mediated eosinophil differentiation and expansion, amd results in a rapid and robust reduction of blood and sputum eosinophil counts. PM1017 is a bispecific antibody Fc-silencing and half -life extension, which targets IL-4Rα and IL-5 simultaneously. This design provides a potential larger effect through the concomitant modulation of both pathways.
-
Dupilumab (anti-IL-4Rα) has been approved for atopic dermatitis with good patient outcomes. However, the effect on inhibiting pruritus is moderate, and more than 50% of patients require improved treatment options. Nemolizumab, which blocks the IL-31/IL-31RA pathway, inhibits pruritus through a direct pathway on sensory nerves, which results in a rapid and robust onset of activity in patients with a reduction of itching and improvement of sleep quality. PM1268 is a bispecific antibody with Fc-silencing and half-life extension, which targets IL-4Rα and IL-31 simultaneously. This design provides a faster onset of efficacy and a potentially larger effect through the concomitant modulation of both pathways.
Gene/Cell Therapy Pipeline






