

Biotheus Shares Latest Research & Development Updates on Multiple Programs During AACR, 2022 and SITC, 2021
- Categories:MEDIA
- Author:
- Origin:
- Time of issue:2022-04-15
- Views:0
(Summary description)Biotheus Inc., participated at the 113th Annual Meeting of the American Association of Cancer Research, 2022, sharing six posters including four bispecifics: PM1022 (PD-L1 x TIGIT), PM1009 (TIGIT x PVRIG), PM4008 (CEACAM5&6 x CD3) and PM1032 (CLDN18.2 x 4-1BB); and two other monoclonal antibodies: PM1038 (CD39) and PM1015 (CD73). Biotheus also previously presented at the Annual SITC meeting in 2021 on programs PM1003 (PD-L1/4-1BB) . Details of these programs are below.
AACR2022
PM1022 (PD-L1 x TIGIT): https://www.abstractsonline.com/pp8/#!/10517/presentation/17744
PM1009 (TIGIT x PVRIG): https://www.abstractsonline.com/pp8/#!/10517/presentation/17746
PM4008 (CEACAM5&6 x CD3): https://www.abstractsonline.com/pp8/#!/10517/presentation/17774
PM1032 (CLDN18.2 x 4-1BB): https://www.abstractsonline.com/pp8/#!/10517/presentation/17775
PM1015 (CD73): https://www.abstractsonline.com/pp8/#!/10517/presentation/18034
PM1038 (CD39): https://www.abstractsonline.com/pp8/#!/10517/presentation/17747
SITC2021
PM1003: http://dx.doi.org/10.1136/jitc-2021-SITC2021.895
BD enquiries and supplemental material requests
Please contact du.yl@biotheus.com or tsun.a@biotheus.com for partnering opportunities and high-resolution PDFs of these posters.
About Biotheus
Founded in 2018, Biotheus focuses on the research and development of next generation multispecific antibodies for oncology and inflammatory diseases. As a clinical-stage biotech company, Biotheus currently has two programs at phase II clinical development and a further six programs at phase I. For more information, please visit www.biotheus.com.
PM1022 (PD-L1 x TIGIT): AACR2022 Abstract #5525
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the most promising 'next generation' immune checkpoint target. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells and promotes the functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. Here, we describe our novel anti-PD-L1 × TIGIT bispecific antibody (PD-L1 × TIGIT biAb) that blocks both the PD-L1/PD-1 and TIGIT/PVR/PVRL2 pathways and has the potential to exhibit equal clinical benefit compared to current combination therapies.
RESULTS: The PD-L1 × TIGIT biAb binds with high affinity to the extracellular domain of human TIGIT and PD-L1 and can bind to TIGIT and PD-L1 simultaneously. In a competition assay, the PD-L1 × TIGIT biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and likewise PD-L1 to PD-1. The PD-L1 × TIGIT biAb induced higher luciferase signals than the anti-TIGIT or anti-PD-L1 mAbs alone in a luciferase reporter-based cell system and enhanced IFN-γ production in an MLR assay. In vivo, the PD-L1 × TIGIT biAb demonstrates similar anti-tumor efficacy to the combination of anti-TIGIT and anti-PD-L1 mAbs, which is stronger than the single-agents alone. We have also completed GLP-toxicity studies that have shown excellent safety. CONCLUSIONS: We have discovered a novel PD-L1 × TIGIT biAb, which induces strong immune responses in vitro and in vivo, supporting its clinical development for the treatment of human cancers. Clinical trials shall be initiating in early 2022.
PM1009 (TIGIT x PVRIG): AACR2022 Abstract #5527
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the promising 'next generation' immune checkpoint molecules. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells, promoting functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. However, the single-agent efficacy of anti-TIGIT therapies have been limited. PVRIG (PVR-related immunoglobulin domain containing), which is another coinhibitory receptor of the DNAM/TIGIT/CD96 nectin family, binds with high affinity to PVRL2 and suppresses T-cell function, and shows nonredundant inhibitory effects alongside the TIGIT/PVR/PVRL2 axis. Here, we report a fully-human anti-TIGIT × PVRIG bispecific antibody (anti-TIGIT × PVRIG biAb), which blocks both the PVRIG/PVRL2 and TIGIT/PVR/PVRL2 pathways, that maintains the efficacy of the combination of the two mono-agents. The anti-TIGIT × PVRIG biAb is also highly efficacious when combined with PD1/PD-L1 inhibitors in mouse tumor models.
RESULTS: The anti-TIGIT × PVRIG biAb binds with high affinity to the extracellular domain of human TIGIT/PVRIG and can bind to TIGIT and PVRIG simultaneously. In a competition assay, the anti-TIGIT × PVRIG biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and PVRIG with PVRL2. In a luciferase reporter cell system, the anti-TIGIT × PVRIG bi
Biotheus Shares Latest Research & Development Updates on Multiple Programs During AACR, 2022 and SITC, 2021
(Summary description)Biotheus Inc., participated at the 113th Annual Meeting of the American Association of Cancer Research, 2022, sharing six posters including four bispecifics: PM1022 (PD-L1 x TIGIT), PM1009 (TIGIT x PVRIG), PM4008 (CEACAM5&6 x CD3) and PM1032 (CLDN18.2 x 4-1BB); and two other monoclonal antibodies: PM1038 (CD39) and PM1015 (CD73). Biotheus also previously presented at the Annual SITC meeting in 2021 on programs PM1003 (PD-L1/4-1BB) . Details of these programs are below.
AACR2022
PM1022 (PD-L1 x TIGIT): https://www.abstractsonline.com/pp8/#!/10517/presentation/17744
PM1009 (TIGIT x PVRIG): https://www.abstractsonline.com/pp8/#!/10517/presentation/17746
PM4008 (CEACAM5&6 x CD3): https://www.abstractsonline.com/pp8/#!/10517/presentation/17774
PM1032 (CLDN18.2 x 4-1BB): https://www.abstractsonline.com/pp8/#!/10517/presentation/17775
PM1015 (CD73): https://www.abstractsonline.com/pp8/#!/10517/presentation/18034
PM1038 (CD39): https://www.abstractsonline.com/pp8/#!/10517/presentation/17747
SITC2021
PM1003: http://dx.doi.org/10.1136/jitc-2021-SITC2021.895
BD enquiries and supplemental material requests
Please contact du.yl@biotheus.com or tsun.a@biotheus.com for partnering opportunities and high-resolution PDFs of these posters.
About Biotheus
Founded in 2018, Biotheus focuses on the research and development of next generation multispecific antibodies for oncology and inflammatory diseases. As a clinical-stage biotech company, Biotheus currently has two programs at phase II clinical development and a further six programs at phase I. For more information, please visit www.biotheus.com.
PM1022 (PD-L1 x TIGIT): AACR2022 Abstract #5525
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the most promising 'next generation' immune checkpoint target. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells and promotes the functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. Here, we describe our novel anti-PD-L1 × TIGIT bispecific antibody (PD-L1 × TIGIT biAb) that blocks both the PD-L1/PD-1 and TIGIT/PVR/PVRL2 pathways and has the potential to exhibit equal clinical benefit compared to current combination therapies.
RESULTS: The PD-L1 × TIGIT biAb binds with high affinity to the extracellular domain of human TIGIT and PD-L1 and can bind to TIGIT and PD-L1 simultaneously. In a competition assay, the PD-L1 × TIGIT biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and likewise PD-L1 to PD-1. The PD-L1 × TIGIT biAb induced higher luciferase signals than the anti-TIGIT or anti-PD-L1 mAbs alone in a luciferase reporter-based cell system and enhanced IFN-γ production in an MLR assay. In vivo, the PD-L1 × TIGIT biAb demonstrates similar anti-tumor efficacy to the combination of anti-TIGIT and anti-PD-L1 mAbs, which is stronger than the single-agents alone. We have also completed GLP-toxicity studies that have shown excellent safety. CONCLUSIONS: We have discovered a novel PD-L1 × TIGIT biAb, which induces strong immune responses in vitro and in vivo, supporting its clinical development for the treatment of human cancers. Clinical trials shall be initiating in early 2022.
PM1009 (TIGIT x PVRIG): AACR2022 Abstract #5527
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the promising 'next generation' immune checkpoint molecules. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells, promoting functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. However, the single-agent efficacy of anti-TIGIT therapies have been limited. PVRIG (PVR-related immunoglobulin domain containing), which is another coinhibitory receptor of the DNAM/TIGIT/CD96 nectin family, binds with high affinity to PVRL2 and suppresses T-cell function, and shows nonredundant inhibitory effects alongside the TIGIT/PVR/PVRL2 axis. Here, we report a fully-human anti-TIGIT × PVRIG bispecific antibody (anti-TIGIT × PVRIG biAb), which blocks both the PVRIG/PVRL2 and TIGIT/PVR/PVRL2 pathways, that maintains the efficacy of the combination of the two mono-agents. The anti-TIGIT × PVRIG biAb is also highly efficacious when combined with PD1/PD-L1 inhibitors in mouse tumor models.
RESULTS: The anti-TIGIT × PVRIG biAb binds with high affinity to the extracellular domain of human TIGIT/PVRIG and can bind to TIGIT and PVRIG simultaneously. In a competition assay, the anti-TIGIT × PVRIG biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and PVRIG with PVRL2. In a luciferase reporter cell system, the anti-TIGIT × PVRIG bi
- Categories:MEDIA
- Author:
- Origin:
- Time of issue:2022-04-15
- Views:0
Biotheus Inc., participated at the 113th Annual Meeting of the American Association of Cancer Research, 2022, sharing six posters including four bispecifics: PM1022 (PD-L1 x TIGIT), PM1009 (TIGIT x PVRIG), PM4008 (CEACAM5&6 x CD3) and PM1032 (CLDN18.2 x 4-1BB); and two other monoclonal antibodies: PM1038 (CD39) and PM1015 (CD73). Biotheus also previously presented at the Annual SITC meeting in 2021 on programs PM1003 (PD-L1/4-1BB) . Details of these programs are below.
AACR2022
PM1022 (PD-L1 x TIGIT): https://www.abstractsonline.com/pp8/#!/10517/presentation/17744
PM1009 (TIGIT x PVRIG): https://www.abstractsonline.com/pp8/#!/10517/presentation/17746
PM4008 (CEACAM5&6 x CD3): https://www.abstractsonline.com/pp8/#!/10517/presentation/17774
PM1032 (CLDN18.2 x 4-1BB): https://www.abstractsonline.com/pp8/#!/10517/presentation/17775
PM1015 (CD73): https://www.abstractsonline.com/pp8/#!/10517/presentation/18034
PM1038 (CD39): https://www.abstractsonline.com/pp8/#!/10517/presentation/17747
SITC2021
PM1003: http://dx.doi.org/10.1136/jitc-2021-SITC2021.895
BD enquiries and supplemental material requests
Please contact du.yl@biotheus.com or tsun.a@biotheus.com for partnering opportunities and high-resolution PDFs of these posters.
About Biotheus
Founded in 2018, Biotheus focuses on the research and development of next generation multispecific antibodies for oncology and inflammatory diseases. As a clinical-stage biotech company, Biotheus currently has two programs at phase II clinical development and a further six programs at phase I. For more information, please visit www.biotheus.com.
PM1022 (PD-L1 x TIGIT): AACR2022 Abstract #5525
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the most promising 'next generation' immune checkpoint target. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells and promotes the functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. Here, we describe our novel anti-PD-L1 × TIGIT bispecific antibody (PD-L1 × TIGIT biAb) that blocks both the PD-L1/PD-1 and TIGIT/PVR/PVRL2 pathways and has the potential to exhibit equal clinical benefit compared to current combination therapies.
RESULTS: The PD-L1 × TIGIT biAb binds with high affinity to the extracellular domain of human TIGIT and PD-L1 and can bind to TIGIT and PD-L1 simultaneously. In a competition assay, the PD-L1 × TIGIT biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and likewise PD-L1 to PD-1. The PD-L1 × TIGIT biAb induced higher luciferase signals than the anti-TIGIT or anti-PD-L1 mAbs alone in a luciferase reporter-based cell system and enhanced IFN-γ production in an MLR assay. In vivo, the PD-L1 × TIGIT biAb demonstrates similar anti-tumor efficacy to the combination of anti-TIGIT and anti-PD-L1 mAbs, which is stronger than the single-agents alone. We have also completed GLP-toxicity studies that have shown excellent safety. CONCLUSIONS: We have discovered a novel PD-L1 × TIGIT biAb, which induces strong immune responses in vitro and in vivo, supporting its clinical development for the treatment of human cancers. Clinical trials shall be initiating in early 2022.
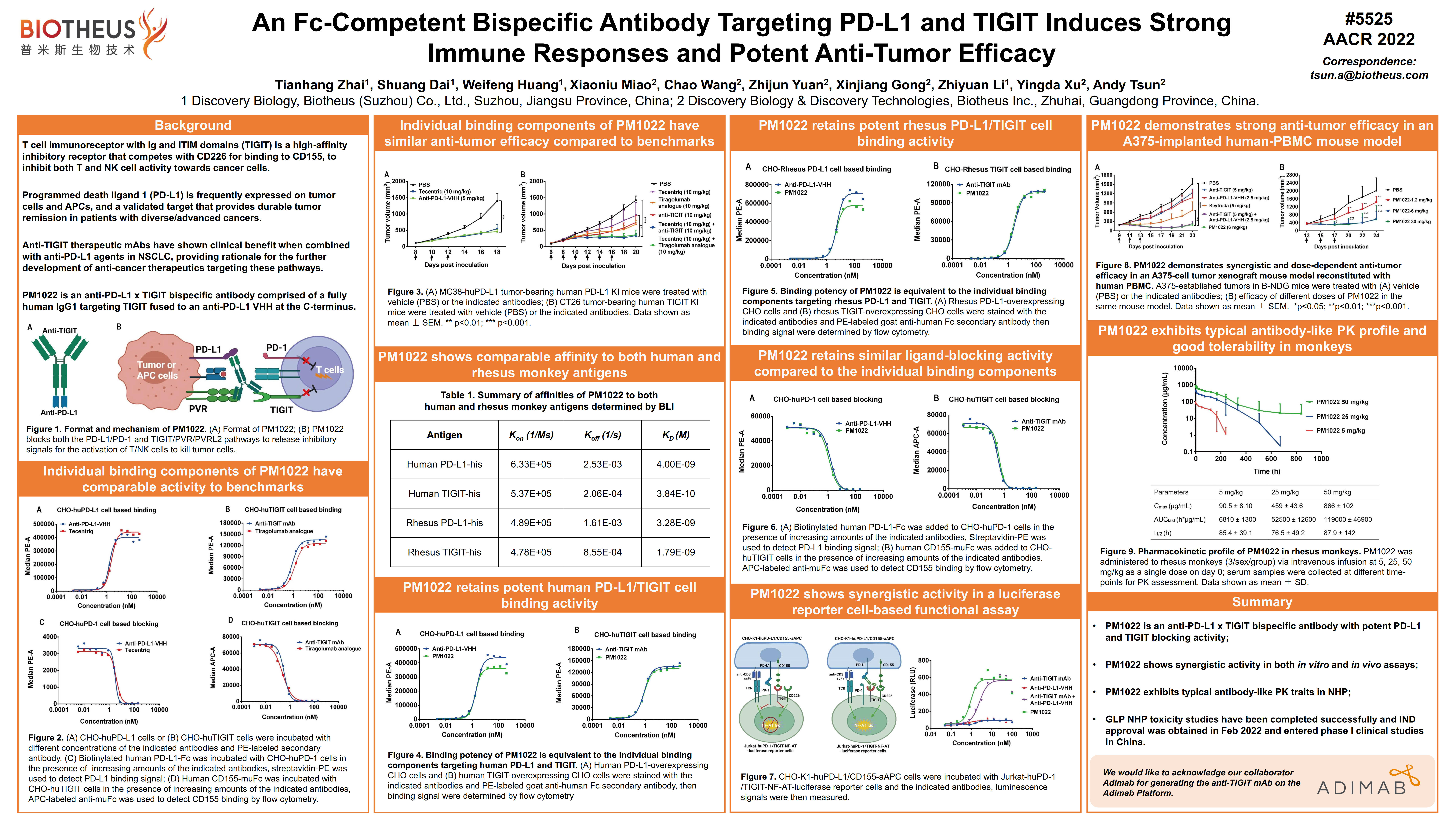
PM1009 (TIGIT x PVRIG): AACR2022 Abstract #5527
BACKGROUND: TIGIT (T-cell immunoglobulin and ITIM domain), which is primarily expressed on activated and 'exhausted' T and NK cells, is one of the promising 'next generation' immune checkpoint molecules. Engagement of TIGIT to its ligands (i.e., PVR and PVRL2) leads to inhibitory signaling in T cells, promoting functional exhaustion of tumor-infiltrating T lymphocytes. Anti-TIGIT monoclonal antibodies have shown clinical benefit when combined with anti-PD-L1 agents in NSCLC. However, the single-agent efficacy of anti-TIGIT therapies have been limited. PVRIG (PVR-related immunoglobulin domain containing), which is another coinhibitory receptor of the DNAM/TIGIT/CD96 nectin family, binds with high affinity to PVRL2 and suppresses T-cell function, and shows nonredundant inhibitory effects alongside the TIGIT/PVR/PVRL2 axis. Here, we report a fully-human anti-TIGIT × PVRIG bispecific antibody (anti-TIGIT × PVRIG biAb), which blocks both the PVRIG/PVRL2 and TIGIT/PVR/PVRL2 pathways, that maintains the efficacy of the combination of the two mono-agents. The anti-TIGIT × PVRIG biAb is also highly efficacious when combined with PD1/PD-L1 inhibitors in mouse tumor models.
RESULTS: The anti-TIGIT × PVRIG biAb binds with high affinity to the extracellular domain of human TIGIT/PVRIG and can bind to TIGIT and PVRIG simultaneously. In a competition assay, the anti-TIGIT × PVRIG biAb efficiently blocked the interaction between TIGIT and PVR/PVRRL2, and PVRIG with PVRL2. In a luciferase reporter cell system, the anti-TIGIT × PVRIG biAb induced high levels of luciferase activity compared with the anti-TIGIT or anti-PVRIG mAbs alone. In vivo, the anti-TIGIT × PVRIG biAb demonstrated stronger anti-tumor efficacy than the anti-TIGIT and anti-PVRIG mAbs as monotherapies or combined with anti-PD-1 mAb. CONCLUSIONS: Our anti-TIGIT × PVRIG biAb, a fully human bispecific antibody, either alone or in combination with anti-PD-1 mAb promotes immune cell activation both in vitro and in vivo, supporting its clinical development for the treatment of human cancers. The molecule is currently under GLP-toxicity evaluation in NHP, and a first-in-human study is expected to begin in 2022.
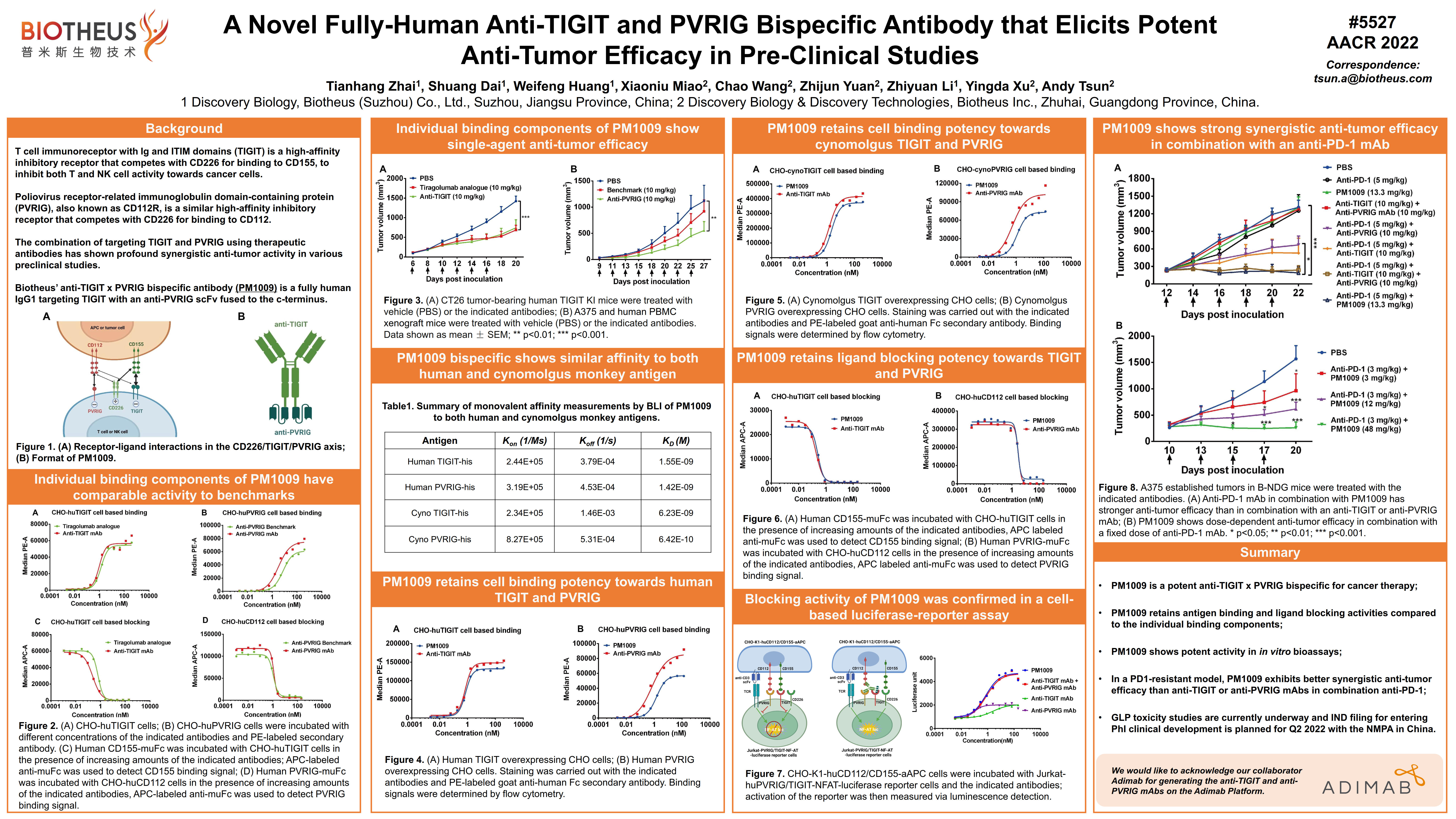
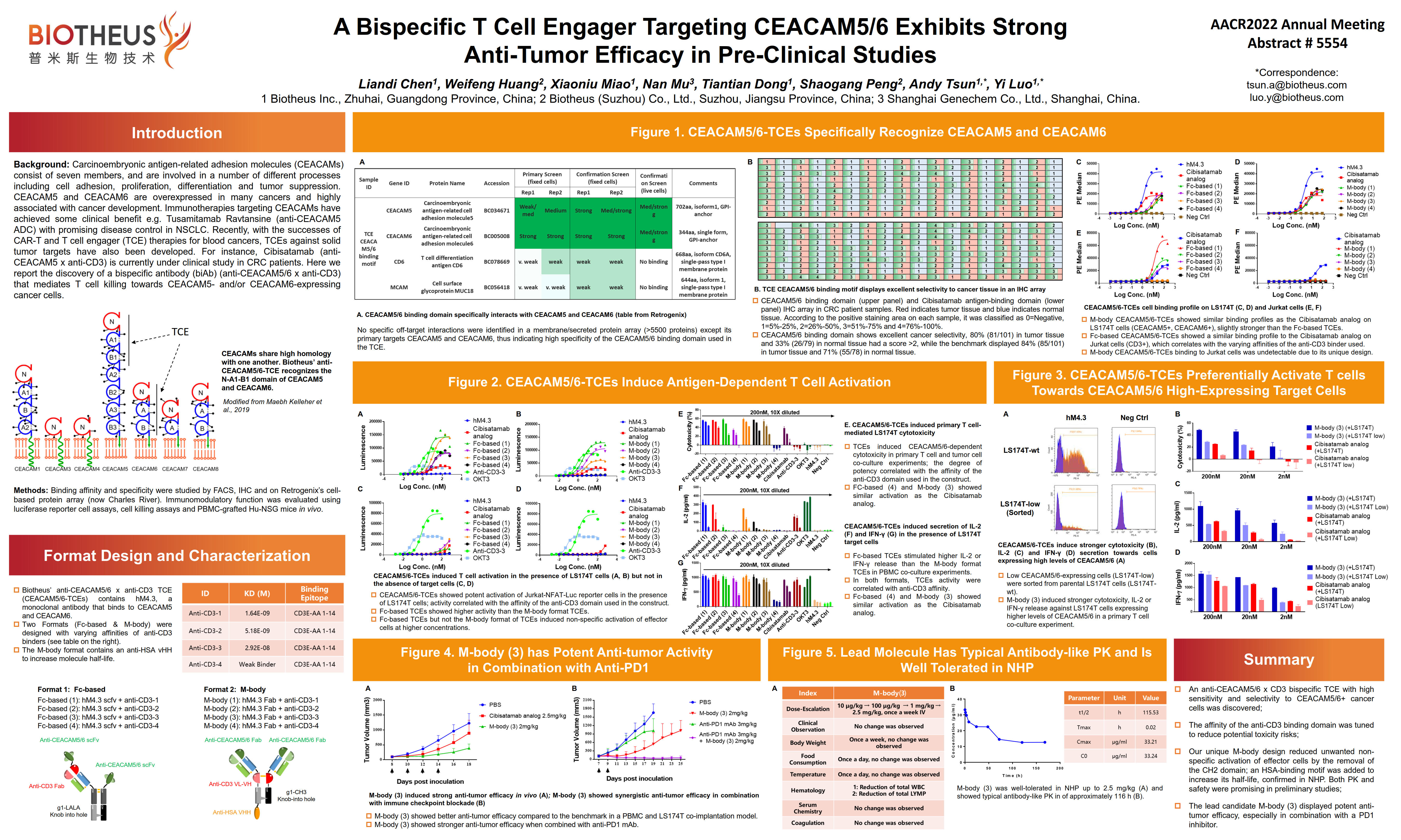
PM4008 (CEACAM5&6 x CD3): AACR2022 Abstract #5554
BACKGROUND: The carcinoembryonic antigen-related adhesion molecules (CEACAM), containing seven members, are involved in many biological processes including cell adhesion, proliferation, differentiation, and tumor suppression. Some variants of CEACAMs such as CEACAM5 and CEACAM6 have long been recognized to be overexpressed in many cancers and highly associated with cancer development. Immunotherapies targeting CEACAM have achieved some clinical benefit, e.g. Tusamitamab Ravtansine (anti-CEACAM5 ADC), that has shown decent disease control in NSCLC. Recently, with the success of CAR-T and T cell engager (TCE) therapies against blood cancers, TCEs against solid tumors have also been developed. For instance, Cibisatamab, targeting CEACAM5 and CD3, is currently under clinical investigation in CRC patients. Here, we report the discovery of a bispecific antibody (biAb) anti-CEACAM5/6 x CD3 that mediates direct T cell killing against CEACAM5- and/or CEACAM6-expressing cancer cells.
RESULTS: Our CEACAM5/6 binding antibody only interacted with CEACAM5 and CEACAM6 on the Retrogenix Cell Microarray against over 5,500 proteins. Importantly, it showed a similar sensitivity to recognize CRC tumor tissues in an IHC array as the benchmark (BMK), but significantly better selectivity at recognition of tumor tissues vs. normal tissues. Next, in vitro testing illustrated CEACAM5/6-mediated T cell activation by the TCE, and such stimulation also correlated with the binding affinity of the anti-CD3 domain. The lead candidate with the optimized CD3 binding affinity, showed better anti-tumor efficacy than the BMK in mice. Furthermore, this molecule displayed a typical PK as a conventional antibody with a mean T1/2 of around 115hrs. Finally, we show high tolerance in NHP of which no obvious signs of toxicity were observed at 2.5 mg/kg. CONCLUSIONS: In summary, a bispecific TCE with high selectivity to CEACAM5/6 overexpressing cancer cells was engineered. It displays potent anti-tumor efficacy, and support from target selectivity testing shows promising safety traits.
PM1032 (CLDN18.2 x 4-1BB): AACR2022 Abstract #5555
BACKGROUND: Gastric cancer is one of the most common cancers worldwide, which is estimated to have 32.1 and 13.2 cases per 100,000 individuals in males and females, respectively. Claudin-18 isoform 2 (CLDN18.2), a membrane-bound protein involved in the formation of tight junctions, has been identified to be highly expressed in various types of cancers such as gastric and pancreatic cancer. Targeting CLDN18.2 with the monoclonal antibody (mAb) Zolbetuximab has achieved moderate clinical benefit with controllable toxicity. 4-1BB is a potent stimulator of T cells and NK cells, and when activated, can improve effector and/or memory responses. However, inherent on-target related toxic effects in the liver was observed during the clinical development of the monoclonal agonist antibody Urelumab. Here, we report the discovery of a humanized bispecific antibody (biAb) anti-CLDN18.2 x 4-1BB, which activates immune cells such as T cells via CLDN18.2-mediated crosslinking of 4-1BB. This bispecific antibody induces potent anti-tumor efficacy with limited toxicity.
RESULTS: The CLDN18.2 antibody interacted specifically to CLDN18.2 on the Retrogenix Cell Microarray platform among over 5,500 proteins. Next, in vitro studies showed how the molecule specifically induced T cell activation and cytokine release against CLDN18.2-positive cells. More importantly, such activation was correlated to the expression level of CLDN18.2, which indicates that the molecule may preferentially recognize CLDN18.2-high-expressing cancer cells. In mouse models, the anti-CLDN18.2 x 4-1BB biAb induced superior anti-tumor efficacy compared to anti-CLDN18.2 and anti-4-1BB mAbs alone with acceptable PK attributes. Furthermore, the anti-CLDN18.2 x 4-1BB biAb induced strong immunological memory where tumors could not grow in re-challenged tumor-free mice. Finally, the CLDN18.2 x 4-1BB biAb showed a safe profile in a GLP NHP study of which no obvious signs of toxicity were observed at 50 mg/kg, 100 mg/kg or 150 mg/kg doses. CONCLUSIONS: In summary, a bispecific antibody targeting CLDN18.2 and 4-1BB was developed, which displayed potent anti-tumor efficacy with strong immunological memory and has shown good safety in NHPs. Moreover, the molecule has high selectivity to CLDN18.2. The molecule shall enter clinical trials by early 2022.

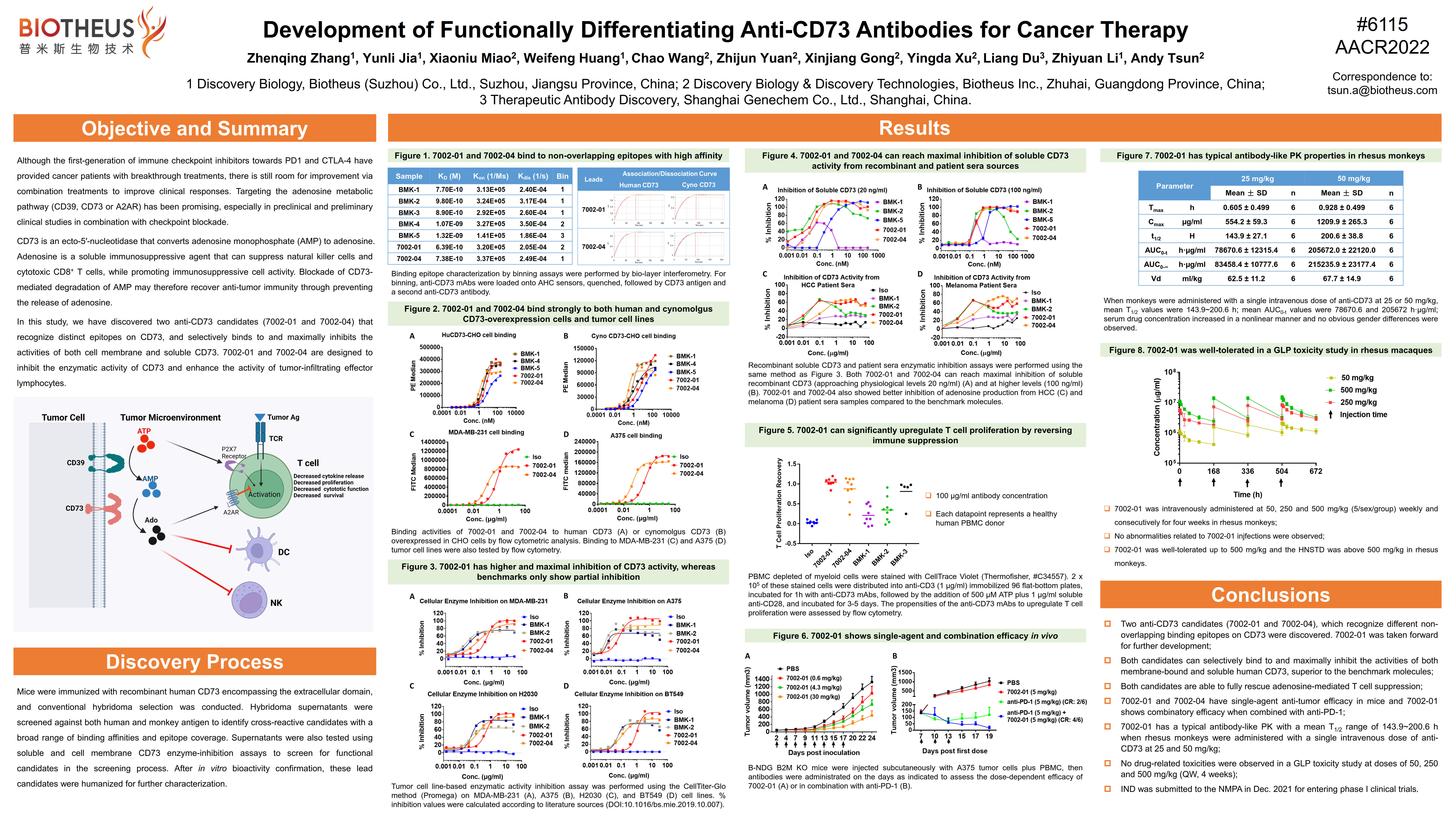
PM1015 (CD73): AACR2022 Abstract #6115
BACKGROUND: Although the discovery and development of first-generation of immune checkpoint inhibitors (towards PD1 and CTLA-4) was a major milestone for cancer therapy, current clinical response rates are still considered very limited. Combination treatments are predicted to improve on these current immunotherapies including the targeting of the adenosine pathway (CD39, CD73 or A2AR), which has shown a lot of promise in preclinical and early clinical studies. CD73 is an ecto-5′-nucleotidase which transforms adenosine monophosphate (AMP) to adenosine. Adenosine is a soluble immunosuppressive metabolite that can suppress natural killer cells and cytotoxic CD8+ T cells. Blockade of CD73-mediated conversion of AMP to adenosine may therefore recover anti-tumor immunity through preventing the enrichment of adenosine in the tumor microenvironment.
RESULTS: Two candidates, 7002-01 and 7002-04, were selected based on their functional activity, that recognize different non-overlapping binding epitopes on CD73. Both candidates selectively bind to and can inhibit the activities of both membrane-bound and soluble human CD73 to high levels and seem to maintain inhibition at high dose-ranges without a hook effect. Both candidates can potently rescue adenosine-mediated T cell regulation. Additionally, 7002-01 and 7002-04 have combination synergy or additive effects for CD73 inhibition on soluble CD73, tumor cell lines that express CD73, and PBMC. 7002-01 and 7002-04 have single-agent anti-tumor efficacy and combination synergy with anti-PD1 antibodies in mice. 7002-01 has a typical antibody-like PK profile when rhesus monkeys were administered with a single intravenous dose at 25 or 50 mg/kg. No drug-related toxicities have been observed in GLP toxicity studies with dosages at 50, 250, and 500 mg/kg (QW, 4 weeks). CONCLUSIONS: Highly differentiating anti-CD73 antibodies were discovered that show maximal inhibition of both membranous and soluble CD73 without hook effects at high concentrations. 7002-01 was chosen as the lead molecule for its better overall activity profile and should be entering clinical trials by early 2022.
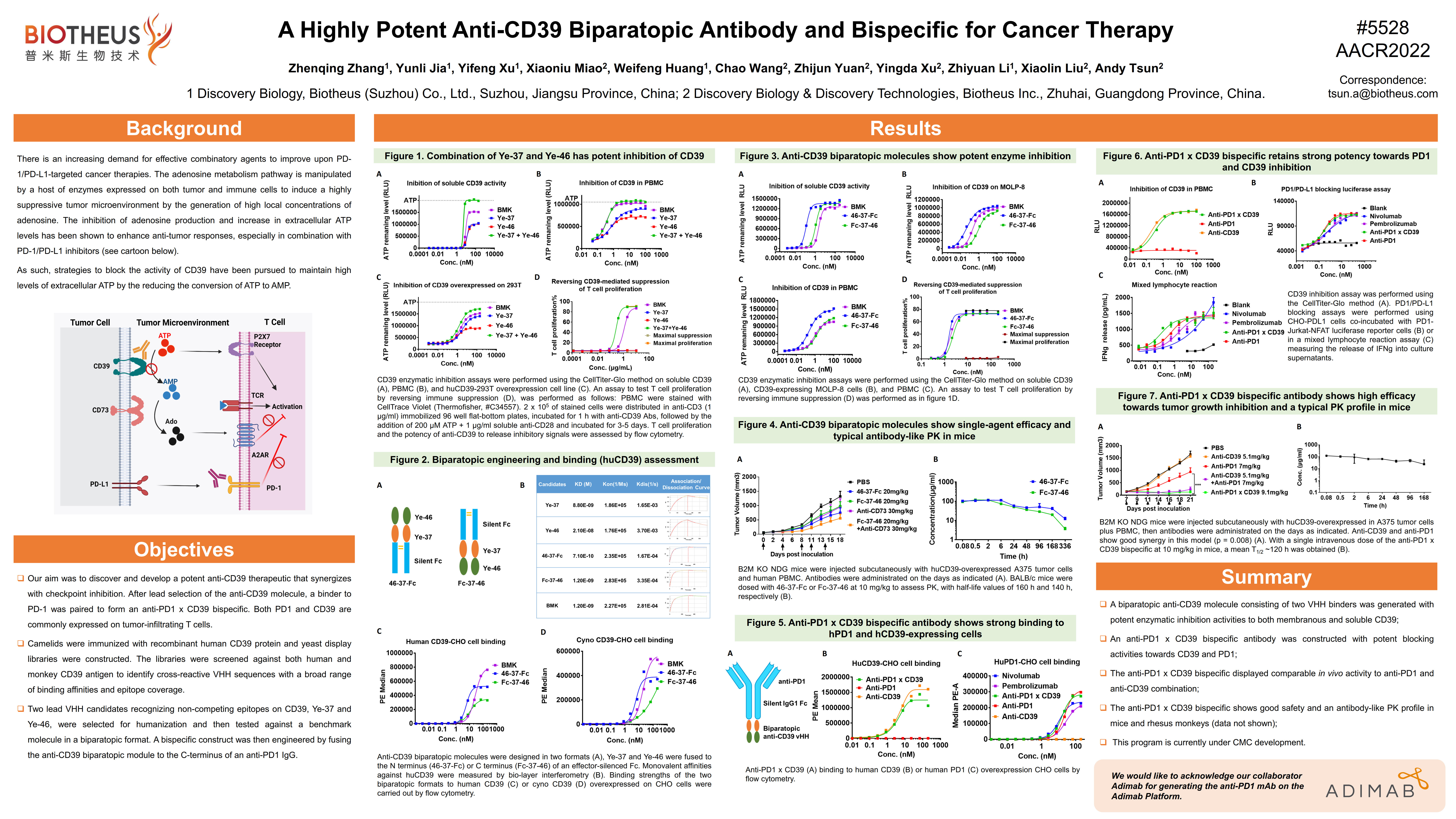
PM1038 (CD39): AACR2022 Abstract #5528
BACKGROUND: There is an increasing demand for effective combinatory agents to improve upon PD-1/PD-L1-based therapeutics. One combinatory target axis is the adenosine metabolism pathway that consists of three major players, including CD39, CD73 and A2AR. Inhibition of any of these targets have shown enhanced preclinical efficacy in combination with PD-1/PD-L1 inhibitors. CD39 is an ectonucleotidase which degrades extracellular ATP to adenosine monophosphate (AMP). This is considered a rate-limiting step for the further degradation to adenosine by CD73. Adenosine is an immunosuppressive metabolite that can suppress NK and T cells. Blockade of CD39-mediated degradation of ATP to AMP may therefore recover anti-tumor immunity through preventing the enrichment of adenosine in the tumor microenvironment.
RESULTS: Two candidates, Ye-37 and Ye-46, were selected for their functional activity that recognize non-overlapping epitopes on CD39. The combination of Ye-37 and Ye-46 shows high potency in cell-based and soluble CD39 assays in blocking CD39 activity. Two biparatopic molecules were generated by fusing the biparatopic unit to the N- or C-termini of Fc (46-37-Fc and Fc-37-46) and showed similar activity to the combination. In vivo, we showed single-agent control of tumor growth and potentiation of tumor-growth inhibition when combined with anti-CD73 antibodies. An anti-PD1 x CD39 bispecific was generated and showed potent inhibition of PD-1/PD-L1 interactions by cell-based assays. Potent anti-tumor efficacy was shown, which was as effective as the combination of anti-PD1 plus anti-CD39 antibodies. CONCLUSIONS: Potent anti-CD39 and anti-PD1 x CD39 therapeutic candidates have been generated with promising activity as a combinatory or single agent, respectively. As such, we plan to file for clinical trial authorization of these programs by 2022.
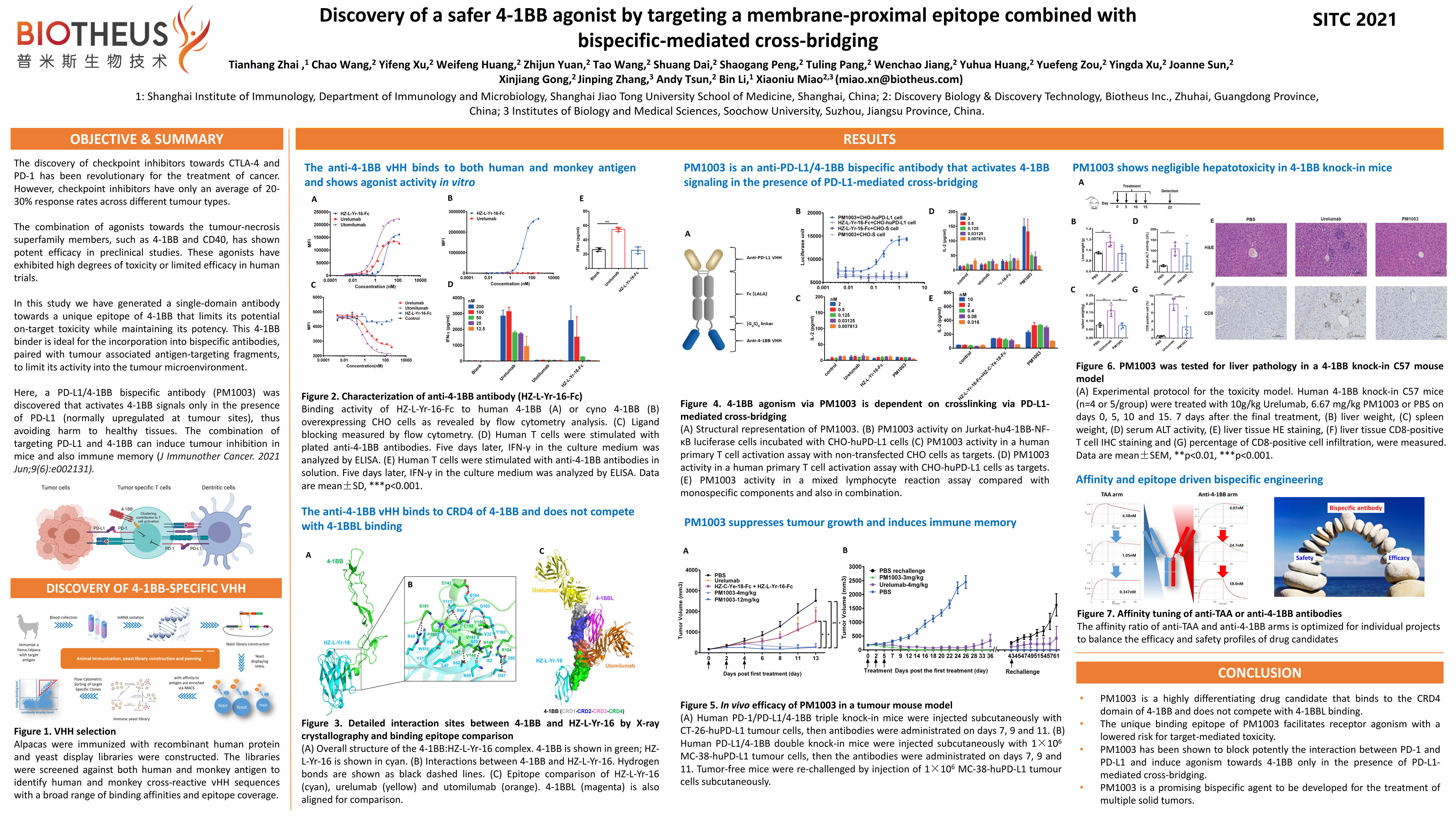
PM1003 (PD-L1 x 4-1BB): SITC2021 Abstract #895
BACKGROUND: Checkpoint inhibitors towards cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have paved the way for a new frontier of anti-cancer therapies that modulate our pre-existing immune system to fight against malignancies. 4-1BB is a tumor-necrosis superfamily member expressed on NK and T cells, that acts as a co-stimulatory receptor to improve effector/memory responses towards tumors. Early efforts have focused on the generation of agonist antibodies towards 4-1BB that relied on Fc-mediated cross-linking to cluster and induce receptor downstream signaling, but has led to liver- and immune-related toxicities. We have discovered a PD-L1 x 4-1BB bispecific that exhibits conditional agonist activity in the presence of PD-L1 with better safety features.
RESULTS: The 4-1BB binder of PM1003 was found to interact with the CRD4 domain of 4-1BB, as determined by X-ray crystallography. Binding to this domain does not affect the binding between 4-1BB and its ligand 4-1BBL. The anti-PD-L1 vHH binds to an epitope on PD-L1 that overlaps with the binding region of PD-1, and is thus effective in disrupting the interaction between PD-1 and PD-L1. Using luciferase reporter assays and primary cell assays we found the PM1003 could activate 4-1BB in the context of PD-L1-mediated cross-bridging. Data from human 4-1BB and PD-L1 knockin mice also showed that PM1003 could effectively control tumor growth without observing any toxicity signals. CONCLUSIONS: PM1003 is a safe and efficacious bispecific antibody that blocks PD-L1 and concurrently activates 4-1BB receptor. An antibody with mild activity was selected directed against the CRD4 domain of 4-1BB to elicit effective potency while minimizing potential toxicity issues. This was reflected in our results. Thus, PM1003 is a potential next generation therapeutic antibody in the IO space that combines and synergizes two independent signaling pathways to control tumor growth.
Scan the QR code to read on your phone
Recommend
- Biotheus Expanded Their Partnership with Hansoh Pharma for Developing EGFR/cMET Bispecific Antibody-Drug Conjugates 2024.03.15
- Biotheus Enters Into Strategic Partnership with BioNTech to Develop and Commercialize Bispecific Antibody Candidate Targeting PD-L1 and VEGF in Multiple Solid Tumor Indications 2023.11.06
- Biotheus Announces Strategic Research Collaboration and Worldwide License Agreement with BioNTech 2023.07.20
- Biotheus has entered into a license and collaboration agreement with Hansoh Pharma for its EGFR/MET bispecific antibody in the Greater China territory 2022.11.15
- Biotheus Shares Latest Research & Development Updates on Multiple Programs During AACR, 2022 and SITC, 2021 2022.04.15
- Biotheus' Bifunctional Therapeutic PM8001 has been Approved by the USFDA to Enter Phase II Studies 2021.12.03



